Role of Next Generation Immune Checkpoint Inhibitor (ICI) Therapy in Philadelphia Negative Classic Myeloproliferative Neoplasm (MPN): Review of the Literature
Abstract
1. Introduction
2. LAG-3 Targeted Therapy and Its Role in Hematological Malignancies
- Use of 89Zr-DFO-REGN3767 in PET scans of people with diffuse large B cell lymphomas (DLBCL) was the pilot study (NCT04566978) [32] undertaken at Memorial Sloan Kettering Hospital in 2022 with the main purpose of the study looking at the way the body absorbs, distributes, and gets rid of 89Zr-DFO-REGN3767 [33]. 89Zr-DFO-REGN3767 is comprised of the anti-LAG-3 antibody, REGN3767 labeled with the positron-emitter zirconium-89 (89Zr) through the chelator-linker DFO and REGN3767 is an investigational monoclonal antibody that targets LAG-3 receptors. This study is a diagnostic research study determining the optimal time for imaging and tumor uptake post 89Zr-DFO-REGN3767 administration. However, it can help evaluate tumor lesion uptake of 89Zr-DFO-REGN3767 and correlate with LAG-3 expression by immunohistochemistry (IHC) in tumors that will be compared descriptively with other biomarkers of TME characterized in biopsies, such as quantitation of IHC score (LAG-3 and/or other immune cell markers).
- A safety and efficacy trial of JCAR017 (lisocabtagene maraleucel, also known as liso-cel) (a CD19-targeted chimeric antigen receptor CART-cell therapy) combinations in subjects with relapsed/refractory B cell malignancies (PLATFORM) (NCT03310619) [34] was performed. Relatlimab, BMS-986016 is an anti-LAG-3 fully human monoclonal IgG4-κ antibody that binds human LAG-3 with high affinity and inhibits its binding to MHC-II [35]. This trial was a global, open-label, multi-arm, parallel multi-cohort, multi-center, Phase I/II study to determine the safety, tolerability, pharmacokinetics, efficacy, and patient-reported quality of life of JCAR017 in combination with various agents including relatlimab, durvalumab, avadomide, iberdomide, ibrutinib, and nivolumab. The trial was completed, and the studied tumors were non-Hodgkin lymphoma (NHL), diffuse large B cell lymphoma (DLBCL), and Follicular lymphoma (FL). The objective of this study was that during Phase I, different arms may be opened to test JCAR017 in combination with combination agent(s) in adult subjects with R/R aggressive B cell NHL. Within each arm, different doses and schedules of JCAR017 and the combination agent(s) may be tested in several cohorts and subcohorts per arm. During Phase II of this study, the expansion of any dose level and schedule for any arm that is safe may occur. All subjects from Phase I and Phase II will be followed for 24 months for survival, relapse, long-term toxicity, and viral vector safety as per guidelines.
- A similar trial was also designed with relatlimab by Bristol-Myers Squibb, NCT02061761 [36] administered alone or in combination with nivolumab to subjects with relapsed or refractory B cell malignancies (relapsed or refractory Hodgkin lymphoma (HL) and relapsed or refractory DLBCL and to study its safety, tolerability, dose-limiting toxicities and maximum tolerated dose. The trial completed and studied hematological malignancies including chronic lymphocytic leukemia (CLL), HL, NHL, and Multiple Myeloma (MM). A detailed description of dose-related adverse events was studied and was +displayed in the result section of the trial.
- Favezelimab (MK-4280) is another LAG-3 antibody that is studied in combination with pembrolizumab (MK-3475) in the clinical trial NCT03598608 [37] that was started in July 2018 to study and evaluate the safety and efficacy of these agents in hematologic malignancies. It included classical HL, DLBCL, and indolent HL. No results have been posted till the writing of this article. This study will also evaluate the safety and efficacy of pembrolizumab or favezelimab administered as monotherapy in participants with classical HL using a 1:1 randomized study design.
- Relapsed or refractory acute myeloid leukemia (AML) and newly diagnosed older AML are included in the ClinicalTrials.gov Identifier: NCT04913922 [38] to study the combination of relatlimab with nivolumab and 5-azacytidine. No results have been posted yet.
3. Mechanism of Action of LAG-3
4. V-Domain Immunoglobulin Suppressor of T Cell Activation (VISTA) Targeted Therapy and Its Role in Hematological Malignancies
5. Role of T Cell Immunoglobulin and Mucin Domain 3 (TIM-3) as Next-Generation ICI in Hematological Malignancies
6. Role of T Cell Immunoglobulin and Immunoreceptor Tyrosine-Based Inhibitory Domain (TIGIT) as a Target for Next-Generation ICI in Hematological Malignancies
7. The Current and Future Role of ICI in the Management of MPN
8. Future Directions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passamonti, F.; Mora, B.; Maffioli, M. New molecular genetics in the diagnosis and treatment of myeloproliferative neoplasms. Curr. Opin. Hematol. 2016, 23, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.G.; Aichberger, K.J.; Luty, S.B.; Bumm, T.G.; Petersen, C.L.; Doratotaj, S.; Vasudevan, K.B.; LaTocha, D.H.; Yang, F.; Press, R.D.; et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood 2011, 118, 6392–6398. [Google Scholar] [CrossRef]
- JJames, C.; Ugo, V.; Le Couédic, J.-P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garçon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef]
- Barosi, G. An Immune Dysregulation in MPN. Curr. Hematol. Malign-Rep. 2014, 9, 331–339. [Google Scholar] [CrossRef]
- Skov, V.; Larsen, T.S.; Thomassen, M.; Riley, C.H.; Jensen, M.K.; Bjerrum, O.W.; Kruse, T.A.; Hasselbalch, H.C. Molecular profiling of peripheral blood cells from patients with polycythemia vera and related neoplasms: Identification of deregulated genes of significance for inflammation and immune surveillance. Leuk. Res. 2012, 36, 1387–1392. [Google Scholar] [CrossRef]
- Zhao, W.B.; Li, Y.; Liu, X.; Zhang, L.Y.; Wang, X. Involvement of CD4+CD25+ regulatory T cells in the pathogenesis of polycythaemia vera. Chin. Med. J. 2008, 121, 1781–1786. [Google Scholar] [CrossRef]
- Skov, V.; Riley, C.H.; Thomassen, M.; Larsen, T.S.; Jensen, M.K.; Bjerrum, O.W.; Kruse, T.A.; Hasselbalch, H.C. Whole blood transcriptional profiling reveals significant down-regulation of human leukocyte antigen class I and II genes in essential thrombocythemia, polycythemia vera and myelofibrosis. Leuk. Lymphoma 2013, 54, 2269–2273. [Google Scholar] [CrossRef]
- Marty, C.; Lacout, C.; Droin, N.; Le Couédic, J.-P.; Ribrag, V.; Solary, E.; Vainchenker, W.; Villeval, J.-L.; Plo, I. A role for reactive oxygen species in JAK2V617F myeloproliferative neoplasm progression. Leukemia 2013, 27, 2187–2195. [Google Scholar] [CrossRef]
- Bjørn, M.E.; Hasselbalch, H.C. The Role of Reactive Oxygen Species in Myelofibrosis and Related Neoplasms. Mediat. Inflamm. 2015, 2015, e648090. [Google Scholar] [CrossRef]
- Wang, J.C.; Kundra, A.; Andrei, M.; Baptiste, S.; Chen, C.; Wong, C.; Sindhu, H. Myeloid-derived suppressor cells in patients with myeloproliferative neoplasm. Leuk. Res. 2016, 43, 39–43. [Google Scholar] [CrossRef]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar] [CrossRef]
- Dalle, I.A.; Kantarjian, H.; Daver, N.; Masarova, L.; Pemmaraju, N.; Bose, P.; Garcia-Manero, G.; Verstovsek, S. Phase II study of single-agent nivolumab in patients with myelofibrosis. Ann. Hematol. 2021, 100, 2957–2960. [Google Scholar] [CrossRef]
- Hobbs, G.; Bozkus, C.C.; Moshier, E.L.; Dougherty, M.; Bar-Natan, M.; Sandy, L.; Johnson, K.; Foster, J.E.; Som, T.; Macrae, M.; et al. PD-1 inhibition in advanced myeloproliferative neoplasms. Blood Adv. 2021, 5, 5086–5097. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes. Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Saleh, R.; Toor, S.M.; Nair, V.S.; Elkord, E. Role of Epigenetic Modifications in Inhibitory Immune Checkpoints in Cancer Development and Progression. Front. Immunol. 2020, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Andreae, S.; Buisson, S.; Triebel, F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood 2003, 102, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347. [Google Scholar] [CrossRef]
- Zuazo, M.; Arasanz, H.; Fernández-Hinojal, G.; García-Granda, M.J.; Gato, M.; Bocanegra, A.; Martínez, M.; Hernández, B.; Teijeira, L.; Morilla, I.; et al. Functional systemic CD 4 immunity is required for clinical responses to PD-L1/ PD-1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef]
- Lichtenegger, F.S.; Rothe, M.; Schnorfeil, F.M.; Deiser, K.; Krupka, C.; Augsberger, C.; Schlüter, M.; Neitz, J.; Subklewe, M. Targeting LAG-3 and PD-1 to Enhance T Cell Activation by Antigen-Presenting Cells. Front. Immunol. 2018, 9, 385. [Google Scholar] [CrossRef]
- Jing, W.; Gershan, J.A.; Weber, J.; Tlomak, D.; McOlash, L.; Sabatos-Peyton, C.; Johnson, B.D. Combined immune checkpoint protein blockade and low dose whole body irradiation as immunotherapy for myeloma. J. Immunother. Cancer 2015, 3, 2. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1–specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical landscape of LAG-3-targeted therapy. Immuno-Oncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb. A Phase 3, Randomized, Double-Blind Study of Adjuvant Immunotherapy with Nivolumab + Relatlimab Fixed-Dose Combination Versus Nivolumab Monotherapy after Complete Resection of Stage III–IV Melanoma. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05002569 (accessed on 9 March 2023).
- Merck Sharp & Dohme LLC. A Phase 3 Study of MK-4280A (Coformulated Favezelimab [MK-4280] Plus Pembrolizumab [MK-3475]) Versus Standard of Care in Previously Treated Metastatic PD-L1 Positive Colorectal Cancer. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05064059 (accessed on 9 March 2023).
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Memorial Sloan Kettering Cancer Center. A Pilot Study of 89Zr-DFO-REGN3767 Anti LAG-3 Antibody Positron Emission Tomography in Patients with Relapsed/Refractory DLBCL. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04566978 (accessed on 12 March 2023).
- Celgene. An Exploratory Phase 1/2 Trial To Evaluate The Safety And Efficacy Of JCAR017 Combinations In Subjects with Relapsed/Refractory B-Cell Malignancies (PLATFORM). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03310619 (accessed on 12 March 2023).
- Albershardt, T.C.; Parsons, A.J.; Reeves, R.S.; Flynn, P.; Campbell, D.J.; ter Meulen, J.; Berglund, P. Therapeutic efficacy of PD1/PDL1 blockade in B16 melanoma is greatly enhanced by immunization with dendritic cell-targeting lentiviral vector and protein vaccine. Vaccine 2020, 38, 3369–3377. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb. A Phase 1/2a Dose Escalation and Cohort Expansion Study of the Safety, Tolerability, and Efficacy of Anti-LAG-3 Monoclonal Antibody (Relatlimab, BMS-986016) Administered Alone and in Combination with Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Relapsed or Refractory B-Cell Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT02061761 (accessed on 12 March 2023).
- Merck Sharp & Dohme LLC. A Phase 1/Phase 2 Clinical Study to Evaluate the Safety and Efficacy of a Combination of MK-4280 and Pembrolizumab (MK-3475) in Participants with Hematologic Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03598608 (accessed on 13 March 2023).
- MD VB. An Open-Label Phase II Study of Relatlimab (BMS-986016) with Nivolumab (BMS-936558) in Combination with 5-Azacytidine for the Treatment of Patients with Refractory/Relapsed Acute Myeloid Leukemia and Newly Diagnosed Older Acute Myeloid Leukemia Patients. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04913922 (accessed on 13 March 2023).
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Wang, J.-H.; Meijers, R.; Xiong, Y.; Liu, J.-H.; Sakihama, T.; Zhang, R.; Joachimiak, A.; Reinherz, E.L. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc. Natl. Acad. Sci. USA 2001, 98, 10799–10804. [Google Scholar] [CrossRef]
- Huard, B.; Mastrangeli, R.; Prigent, P.; Bruniquel, D.; Donini, S.; El-Tayar, N.; Maigret, B.; Dréano, M.; Triebel, F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl. Acad. Sci. USA 1997, 94, 5744–5749. [Google Scholar] [CrossRef]
- Hannier, S.; Triebel, F. The MHC class II ligand lymphocyte activation gene-3 is co-distributed with CD8 and CD3-TCR molecules after their engagement by mAb or peptide-MHC class I complexes. Int. Immunol. 1999, 11, 1745–1752. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Micangeli, E.; Donini, S. Cloning of Murine LAG-3 by Magnetic Bead Bound Homologous Probes and PCR (GENE-CAPTURE PCR). Anal. Biochem. 1996, 241, 93–102. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Ruiter, D.J.; Mattijssen, V.; Broecker, E.B.; Ferrone, S. MHC antigens in human melanomas. Semin. Cancer Biol. 1991, 2, 35–45. [Google Scholar]
- Hemon, P.; Jean-Louis, F.; Ramgolam, K.; Brignone, C.; Viguier, M.; Bachelez, H.; Triebel, F.; Charron, D.; Aoudjit, F.; Al-Daccak, R.; et al. MHC Class II Engagement by Its Ligand LAG-3 (CD223) Contributes to Melanoma Resistance to Apoptosis. J. Immunol. 2011, 186, 5173–5183. [Google Scholar] [CrossRef]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T-Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-cell Responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Wang, Y.; El Kasmi, K.C.; Pardoll, D.M.; Murray, P.J.; Drake, C.G.; Vignali, D.A.A. LAG-3 Regulates Plasmacytoid Dendritic Cell Homeostasis. J. Immunol. 2009, 182, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Camisaschi, C.; De Filippo, A.; Beretta, V.; Vergani, B.; Villa, A.; Vergani, E.; Santinami, M.; Cabras, A.D.; Arienti, F.; Triebel, F.; et al. Alternative Activation of Human Plasmacytoid DCs In Vitro and in Melanoma Lesions: Involvement of LAG-3. J. Investig. Dermatol. 2014, 134, 1893–1902. [Google Scholar] [CrossRef]
- Huang, B.; Lei, Z.; Zhao, J.; Gong, W.; Liu, J.; Chen, Z.; Liu, Y.; Li, D.; Yuan, Y.; Zhang, G.-M.; et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007, 252, 86–92. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; Black, R.A.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.-F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef]
- Borggrewe, M.; Grit, C.; Den Dunnen, W.F.A.; Burm, S.M.; Bajramovic, J.J.; Noelle, R.J.; Eggen, B.J.L.; Laman, J.D. VISTA expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia 2018, 66, 2645–2658. [Google Scholar] [CrossRef]
- ElTanbouly, M.; Croteau, W.; Noelle, R.J.; Lines, J.L. VISTA: A novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 2019, 42, 101308. [Google Scholar] [CrossRef]
- Flies, D.B.; Wang, S.; Xu, H.; Chen, L. Cutting Edge: A Monoclonal Antibody Specific for the Programmed Death-1 Homolog Prevents Graft-versus-Host Disease in Mouse Models. J. Immunol. 2011, 187, 1537–1541. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Schaafsma, E.; Noelle, R.J.; Lines, J.L. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin. Exp. Immunol. 2020, 200, 120–130. [Google Scholar] [CrossRef]
- Blando, J.; Sharma, A.; Higa, M.G.; Zhao, H.; Vence, L.; Yadav, S.S.; Kim, J.; Sepulveda, A.M.; Sharp, M.; Maitra, A.; et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 1692–1697. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Knecht, M.; Mollaee, M.; Zhong, Z.; Erkes, D.A.; McCue, P.A.; Chervoneva, I.; Berger, A.C.; Lo, J.A.; Fisher, D.E.; et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020, 30, 510–524. [Google Scholar] [CrossRef]
- Kuklinski, L.F.; Yan, S.; Li, Z.; Fisher, J.L.; Cheng, C.; Noelle, R.J.; Angeles, C.V.; Turk, M.J.; Ernstoff, M.S. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol. Immunother. 2018, 67, 1113–1121. [Google Scholar] [CrossRef]
- Wang, L.; Jia, B.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; Mineishi, S.; Naik, S.; Khawaja, M.R.; Sivik, J.; Han, J.; et al. VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. Oncoimmunology 2018, 7, e1469594. [Google Scholar] [CrossRef]
- Aru, B.; Pehlivanoğlu, C.; Dal, Z.; Dereli-Çalışkan, N.N.; Gürlü, E.; Yanıkkaya-Demirel, G. A potential area of use for immune checkpoint inhibitors: Targeting bone marrow microenvironment in acute myeloid leukemia. Front. Immunol. 2023, 14, 1108200. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Lines, J.L.; Sempere, L.F.; Broughton, T.; Wang, L.; Noelle, R. VISTA Is a Novel Broad-Spectrum Negative Checkpoint Regulator for Cancer Immunotherapy. Cancer Immunol. Res. 2014, 2, 510–517. [Google Scholar] [CrossRef]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor–immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Loeser, H.; Kraemer, M.; Gebauer, F.; Bruns, C.; Schröder, W.; Zander, T.; Persa, O.-D.; Alakus, H.; Hoelscher, A.; Buettner, R.; et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology 2019, 8, e1581546. [Google Scholar] [CrossRef] [PubMed]
- Janssen Research & Development, LLC. An Open-Label, First-in-Human, Phase 1 Study of the Safety, Pharmacokinetics, and Pharmacodynamics of JNJ-61610588, a Fully Human IgG1 Kappa Anti-VISTA (V-Domain Ig Suppressor of T-Cell Activation) Monoclonal Antibody, in Subjects with Advanced Cancer. clinicaltrials.gov. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02671955 (accessed on 16 March 2023).
- Curis, Inc. A Phase 1, Open-Label, Dose Escalation and Dose Expansion Trial Evaluating the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Effects of Orally Administered CA-170 in Patients with Advanced Tumors and Lymphomas. clinicaltrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02812875 (accessed on 16 March 2023).
- Wu, C.; Cao, X.; Zhang, X. VISTA inhibitors in cancer immunotherapy: A short perspective on recent progresses. RSC Med. Chem. 2021, 12, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Ingram, P.J.; Thakkar, D.; Boyd-Kirkup, J.D. Abstract 587: HMBD002, a novel neutralizing antibody targeting a specific epitope on the co-inhibitory immune checkpoint receptor VISTA, displays potent anti-tumor effects in pre-clinical models. Cancer Res. 2017, 77, 587. [Google Scholar] [CrossRef]
- Gou, R.; Zhu, L.; Zheng, M.; Guo, Q.; Hu, Y.; Li, X.; Liu, J.; Lin, B. Annexin A8 can serve as potential prognostic biomarker and therapeutic target for ovarian cancer: Based on the comprehensive analysis of Annexins. J. Transl. Med. 2019, 17, 275. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Ferris, R.L.; Lu, B.; Kane, L.P. Too Much of a Good Thing? Tim-3 and TCR Signaling in T Cell Exhaustion. J. Immunol. 2014, 193, 1525–1530. [Google Scholar] [CrossRef]
- Phong, B.L.; Avery, L.; Sumpter, T.L.; Gorman, J.V.; Watkins, S.C.; Colgan, J.D.; Kane, L.P. Tim-3 enhances FcεRI-proximal signaling to modulate mast cell activation. J. Exp. Med. 2015, 212, 2289–2304. [Google Scholar] [CrossRef]
- Baitsch, L.; Baumgaertner, P.; Devêvre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011, 121, 2350–2360. [Google Scholar] [CrossRef]
- Kikushige, Y.; Shima, T.; Takayanagi, S.-I.; Urata, S.; Miyamoto, T.; Iwasaki, H.; Takenaka, K.; Teshima, T.; Tanaka, T.; Inagaki, Y.; et al. TIM-3 Is a Promising Target to Selectively Kill Acute Myeloid Leukemia Stem Cells. Cell Stem Cell 2010, 7, 708–717. [Google Scholar] [CrossRef]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of Tissue Inflammation by the Immune Receptor Tim-3 Expressed on Innate Immune Cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.J.; Wang, J.M.; Ji, X.J.; Wu, X.Y.; Jia, Z.S.; Moorman, J.P.; Yao, Z.Q. Tim-3 Negatively Regulates IL-12 Expression by Monocytes in HCV Infection. PLoS ONE 2011, 6, e19664. [Google Scholar] [CrossRef]
- Maurya, N.; Gujar, R.; Gupta, M.; Yadav, V.; Verma, S.; Sen, P. Immunoregulation of Dendritic Cells by the Receptor T cell Ig and Mucin Protein-3 via Bruton’s Tyrosine Kinase and c-Src. J. Immunol. 2014, 193, 3417–3425. [Google Scholar] [CrossRef]
- Su, E.W.; Bi, S.; Kane, L.P. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology 2010, 21, 1258–1265. [Google Scholar] [CrossRef]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef]
- DeKruyff, R.H.; Bu, X.; Ballesteros, A.; Santiago, C.; Chim, Y.-L.E.; Lee, H.-H.; Karisola, P.; Pichavant, M.; Kaplan, G.G.; Umetsu, D.T.; et al. T Cell/Transmembrane, Ig, and Mucin-3 Allelic Variants Differentially Recognize Phosphatidylserine and Mediate Phagocytosis of Apoptotic Cells. J. Immunol. 2010, 184, 1918–1930. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.F.; Dougan, S.K.; Petersen, B.-S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef]
- Rangachari, M.; Zhu, C.; Sakuishi, K.; Xiao, S.; Karman, J.; Chen, A.; Angin, M.; Wakeham, A.; Greenfield, E.A.; Sobel, R.A.; et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion. Nat. Med. 2012, 18, 1394–1400. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef]
- Tao, J.-L.; Li, L.-J.; Fu, R.; Wang, H.-Q.; Jiang, H.-J.; Yue, L.-Z.; Zhang, W.; Liu, H.; Ruan, E.-B.; Qu, W.; et al. Elevated TIM3+ hematopoietic stem cells in untreated myelodysplastic syndrome displayed aberrant differentiation, overproliferation and decreased apoptosis. Leuk. Res. 2014, 38, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Tcvetkov, N.; Gusak, A.; Morozova, E.; Moiseev, I.; Baykov, V.; Barabanshikova, M.; Lepik, K.; Bakin, E.; Vlasova, J.; Osipova, A.; et al. Immune checkpoints bone marrow expression as the predictor of clinical outcome in myelodysplastic syndrome. Leuk. Res. Rep. 2020, 14, 100215. [Google Scholar] [CrossRef] [PubMed]
- Asayama, T.; Tamura, H.; Ishibashi, M.; Kuribayashi-Hamada, Y.; Onodera-Kondo, A.; Okuyama, N.; Yamada, A.; Shimizu, M.; Moriya, K.; Takahashi, H.; et al. Functional expression of Tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget 2017, 8, 88904–88917. [Google Scholar] [CrossRef] [PubMed]
- Brück, O.; Blom, S.; Dufva, O.; Turkki, R.; Chheda, H.; Ribeiro, A.; Kovanen, P.; Aittokallio, T.; Koskenvesa, P.; Kallioniemi, O.; et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia 2018, 32, 1643–1656. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, G.; Zhou, S.; He, X.; Sun, N.; Zhu, X.; Hu, A. Blocking Tim-3 or/and PD-1 reverses dysfunction of tumor-infiltrating lymphocytes in HBV-related hepatocellular carcinoma. Bull. Cancer 2018, 105, 493–501. [Google Scholar] [CrossRef]
- Schnell, A.; Bod, L.; Madi, A.; Kuchroo, V.K. The yin and yang of co-inhibitory receptors: Toward anti-tumor immunity without autoimmunity. Cell Res. 2020, 30, 285–299. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Chen, Z. Tim-3 expression and its role in hepatocellular carcinoma. J. Hematol. Oncol. 2018, 11, 126. [Google Scholar] [CrossRef]
- Tan, J.; Huang, S.; Huang, J.; Yu, Z.; Chen, Y.; Lu, Y.; Li, Y.; Chen, S. Increasing Tim-3+CD244+, Tim-3+CD57+, and Tim-3+PD-1+ T cells in patients with acute myeloid leukemia. Asia-Pacific J. Clin. Oncol. 2020, 16, 137–141. [Google Scholar] [CrossRef]
- Hadadi, L.; Hafezi, M.; Amirzargar, A.A.; Sharifian, R.A.; Abediankenari, S.; Asgarian-Omran, H. Dysregulated Expression of Tim-3 and NKp30 Receptors on NK Cells of Patients with Chronic Lymphocytic Leukemia. Oncol. Res. Treat. 2019, 42, 197–203. [Google Scholar] [CrossRef]
- Rezazadeh, H.; Astaneh, M.; Tehrani, M.; Hossein-Nataj, H.; Zaboli, E.; Shekarriz, R.; Asgarian-Omran, H. Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8+ T cells in early clinical stages of chronic lymphocytic leukemia. Immunol. Res. 2020, 68, 269–279. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Komrokji, R.S.; Brunner, A.M. TIM-3 pathway dysregulation and targeting in cancer. Expert. Rev. Anticancer. Ther. 2021, 21, 523–534. [Google Scholar] [CrossRef]
- Harding, J.J.; Moreno, V.; Bang, Y.-J.; Hong, M.H.; Patnaik, A.; Trigo, J.; Szpurka, A.M.; Yamamoto, N.; Doi, T.; Fu, S.; et al. Blocking TIM-3 in Treatment-refractory Advanced Solid Tumors: A Phase Ia/b Study of LY3321367 with or without an Anti-PD-L1 Antibody. Clin. Cancer Res. 2021, 27, 2168–2178. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. Phase 1b, Multi-Arm, Open-Label Study of PDR001 and/or MBG453 in Combination with Decitabine in Patients with Acute Myeloid Leukemia or High Risk Myelodysplastic Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT03066648 (accessed on 12 May 2023).
- Bewersdorf, J.P.; Stahl, M.; Zeidan, A.M. Immune checkpoint-based therapy in myeloid malignancies: A promise yet to be fulfilled. Expert. Rev. Anticancer Ther. 2019, 19, 393–404. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Chan, C.J.; Andrews, D.M.; Smyth, M.J. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr. Opin. Immunol. 2012, 24, 246–251. [Google Scholar] [CrossRef]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef]
- Mendelsohn, C.L.; Wimmer, E.; Racaniello, V.R. Cellular receptor for poliovirus: Molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 1989, 56, 855–865. [Google Scholar] [CrossRef]
- Eberlé, F.; Dubreuil, P.; Mattei, M.-G.; Devilard, E.; Lopez, M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 1995, 159, 267–272. [Google Scholar] [CrossRef]
- Reymond, N.; Borg, J.-P.; Lecocq, E.; Adelaide, J.; Campadelli-Fiume, G.; Dubreuil, P.; Lopez, M. Human nectin3/PRR3: A novel member of the PVR/PRR/nectin family that interacts with afadin. Gene 2000, 255, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8 + T Cell Effector Function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Dominguez-Villar, M.; Kuchroo, V.; Hafler, D.A. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 2012, 188, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhu, L.; Schell, T.D.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; George, M.R.; Zeng, H.; Zheng, H. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clin. Cancer Res. 2016, 22, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.-H.T.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Guillerey, C.; Harjunpää, H.; Carrié, N.; Kassem, S.; Teo, T.; Miles, K.; Krumeich, S.; Weulersse, M.; Cuisinier, M.; Stannard, K.; et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood 2018, 132, 1689–1694. [Google Scholar] [CrossRef]
- Stålhammar, G.; Seregard, S.; Grossniklaus, H.E. Expression of immune checkpoint receptors Indoleamine 2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus nonmetastatic choroidal melanoma. Cancer Med. 2019, 8, 2784–2792. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Klampatsa, A.; Thompson, J.C.; Martinez, M.C.; Hwang, W.-T.; Rao, A.S.; Standalick, J.E.; Kim, S.; Cantu, E.; Litzky, L.A.; et al. Function of Human Tumor-Infiltrating Lymphocytes in Early-Stage Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2019, 7, 896–909. [Google Scholar] [CrossRef]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Degos, C.; Heinemann, M.; Barrou, J.; Boucherit, N.; Lambaudie, E.; Savina, A.; Gorvel, L.; Olive, D. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front. Immunol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Josefsson, S.E.; Huse, K.; Kolstad, A.; Beiske, K.; Pende, D.; Steen, C.B.; Inderberg, E.M.; Lingjærde, O.C.; Østenstad, B.; Smeland, E.B.; et al. T Cells Expressing Checkpoint Receptor TIGIT Are Enriched in Follicular Lymphoma Tumors and Characterized by Reversible Suppression of T-cell Receptor Signaling. Clin. Cancer Res. 2018, 24, 870–881. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Chauvin, J.-M.; Ka, M.; Davar, D.; Pagliano, O.; Wang, H.; Saada, S.; Menna, C.; Amin, R.; et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. J. Clin. Investig. 2018, 3, 121157. [Google Scholar] [CrossRef]
- Dixon, K.O.; Schorer, M.; Nevin, J.; Etminan, Y.; Amoozgar, Z.; Kondo, T.; Kurtulus, S.; Kassam, N.; Sobel, R.A.; Fukumura, D.; et al. Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity. J. Immunol. 2018, 200, 3000–3007. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef]
- Wu, A.A.; Drake, V.; Huang, H.-S.; Chiu, S.; Zheng, L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015, 4, e1016700. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef]
- Harjunpää, H.; Blake, S.J.; Ahern, E.; Allen, S.; Liu, J.; Yan, J.; Lutzky, V.; Takeda, K.; Aguilera, A.R.; Guillerey, C.; et al. Deficiency of host CD96 and PD-1 or TIGIT enhances tumor immunity without significantly compromising immune homeostasis. Oncoimmunology 2018, 7, e1445949. [Google Scholar] [CrossRef]
- Genentech, Inc. A Phase II, Randomized, Blinded, Placebo-Controlled Study of Tiragolumab, An Anti-TIGIT Antibody, In Combination with Atezolizumab In Chemotherapy-Naïve Patients with Locally Advanced Or Metastatic Non-Small Cell Lung Cancer. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03563716 (accessed on 16 March 2023).
- Arcus Biosciences, Inc. A Phase 1 Study to Evaluate the Safety and Tolerability of AB154 Monotherapy and Combination Therapy in Participants with Advanced Malignancies. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03628677 (accessed on 16 March 2023).
- Merck Sharp & Dohme LLC. A Phase 1 Trial of MK-7684 as Monotherapy and in Combination with Pembrolizumab in Subjects with Advanced Solid Tumors. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02964013 (accessed on 16 March 2023).
- Bristol-Myers Squibb. Phase 1/2a First-In-Human Study of BMS-986207 Monoclonal Antibody Alone and in Combination with Nivolumab or with Nivolumab and Ipilimumab in Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02913313 (accessed on 16 March 2023).
- Astellas Pharma Global Development, Inc. A Phase 1b Study of ASP8374, an Immune Checkpoint Inhibitor, as a Single Agent and in Combination with Pembrolizumab in Subjects with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03260322 (accessed on 16 March 2023).
- Astellas Pharma Inc. A Phase 1, Open Label Study of ASP8374, an Immune Checkpoint Inhibitor, in Japanese Patients with Advanced Solid Tumors. clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03945253 (accessed on 16 March 2023).
- Jin, S.; Zhang, Y.; Zhou, F.; Chen, X.; Sheng, J.; Zhang, J. TIGIT: A promising target to overcome the barrier of immunotherapy in hematological malignancies. Front. Oncol. 2022, 12, 1091782. [Google Scholar] [CrossRef] [PubMed]
- Catakovic, K.; Gassner, F.J.; Ratswohl, C.; Zaborsky, N.; Rebhandl, S.; Schubert, M.; Steiner, M.; Gutjahr, J.C.; Pleyer, L.; Egle, A.; et al. TIGIT expressing CD4+T cells represent a tumor-supportive T cell subset in chronic lymphocytic leukemia. Oncoimmunology 2017, 7, e1371399. [Google Scholar] [CrossRef] [PubMed]
- Minnie, S.A.; Kuns, R.D.; Gartlan, K.H.; Zhang, P.; Wilkinson, A.N.; Samson, L.; Guillerey, C.; Engwerda, C.; MacDonald, K.P.A.; Smyth, M.J.; et al. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood 2018, 132, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-La Roche. A Phase Ib/II Open-Label, Multicenter Study Evaluating the Safety, Efficacy, and Pharmacokinetics of Mosunetuzumab in Combination with Tiragolumab with or without Atezolizumab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05315713 (accessed on 12 May 2023).
- Genentech, Inc. A Phase Ia/Ib Open-Label, Multicenter Study Evaluating the Safety and Pharmacokinetics of Tiragolumab as a Single Agent and in Combination with Atezolizumab and/or Daratumumab in Patients with Relapsed or Refractory Multiple Myeloma, and as a Single Agent and in Combination with Rituximab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04045028 (accessed on 12 May 2023).
- BeiGene. A Phase 1b/2 Study Investigating the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of Ociperlimab (BGB A1217) in Combination with Tislelizumab (BGB A317) or Rituximab in Patients with Relapsed or Refractory Diffuse Large B Cell Lymphoma. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05267054 (accessed on 12 May 2023).
- Multiple Myeloma Research Consortium. A Phase I/II Assessment of Combination Immuno-Oncology Drugs Elotuzumab, Anti-LAG-3 (BMS-986016) and Anti-TIGIT (BMS-986207). clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04150965 (accessed on 12 May 2023).
- Merck Sharp & Dohme LLC. A Phase 2, Open-Label Study to Evaluate the Safety and Efficacy of MK-7684A (MK-7684 [Vibostolimab] with MK-3475 [Pembrolizumab] Coformulation) in Participants with Relapsed or Refractory Hematological Malignancies. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05005442 (accessed on 12 May 2023).
- Compugen Ltd. A Phase 1 Study of The Safety and Tolerability of COM902 in Subjects with Advanced Malignancies. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04354246 (accessed on 12 May 2023).
- Seagen Inc. A Phase 1 Study of SEA-TGT (SGN-TGT) in Subjects with Advanced Malignancies. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04254107 (accessed on 12 May 2023).
- Arcus Biosciences, Inc. A Phase 1/1b Study to Evaluate the Safety and Tolerability of AB308 in Combination with AB122 in Participants with Advanced Malignancies. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04772989 (accessed on 12 May 2023).
- iTeos Belgium SA. Study of EOS884448 Alone, and in Combination with Iberdomide with or without Dexamethasone, in Participants with Relapsed or Refractory Multiple Myeloma. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05289492 (accessed on 12 May 2023).
- Barbui, T.; Barosi, G.; Birgegard, G.; Cervantes, F.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.C.; Hehlmann, R.; Hoffman, R.; et al. Philadelphia-Negative Classical Myeloproliferative Neoplasms: Critical Concepts and Management Recommendations from European LeukemiaNet. J. Clin. Oncol. 2011, 29, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Verstovsek, S. The Rationale for Immunotherapy in Myeloproliferative Neoplasms. Curr. Hematol. Malign-Rep. 2019, 14, 310–327. [Google Scholar] [CrossRef]
- Barbui, T.; Vannucchi, A.M.; Buxhofer-Ausch, V.; De Stefano, V.; Betti, S.; Rambaldi, A.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015, 5, e369. [Google Scholar] [CrossRef]
- Alvarez-Larrán, A.; Garrote, M.; Ferrer-Marín, F.; Pérez-Encinas, M.; Mata-Vazquez, M.I.; Bellosillo, B.; Arellano-Rodrigo, E.; Gómez, M.; García, R.; García-Gutiérrez, V.; et al. Real-world analysis of main clinical outcomes in patients with polycythemia vera treated with ruxolitinib or best available therapy after developing resistance/intolerance to hydroxyurea. Cancer 2022, 128, 2441–2448. [Google Scholar] [CrossRef]
- Wagner, S.M.; Melchardt, T.; Greil, R. Ropeginterferon alfa-2b for the treatment of patients with polycythemia vera. Drugs Today 2020, 56, 195–202. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Mesa, R.; et al. Ruxolitinib versus Standard Therapy for the Treatment of Polycythemia Vera. N. Engl. J. Med. 2015, 372, 426–435. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Hoffman, R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood 2013, 121, 4832–4837. [Google Scholar] [CrossRef]
- Kvasnicka, H.M.; Thiele, J.; Bueso-Ramos, C.E.; Sun, W.; Cortes, J.; Kantarjian, H.M.; Verstovsek, S. Long-term effects of ruxolitinib versus best available therapy on bone marrow fibrosis in patients with myelofibrosis. J. Hematol. Oncol. 2018, 11, 42. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Verstovsek, S.; Guglielmelli, P.; Griesshammer, M.; Burn, T.C.; Naim, A.; Paranagama, D.; Marker, M.; Gadbaw, B.; Kiladjian, J.-J. Ruxolitinib reduces JAK2 p.V617F allele burden in patients with polycythemia vera enrolled in the RESPONSE study. Ann. Hematol. 2017, 96, 1113–1120. [Google Scholar] [CrossRef]
- Deininger, M.; Radich, J.; Burn, T.C.; Huber, R.; Paranagama, D.; Verstovsek, S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood 2015, 126, 1551–1554. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Kantarjian, H.M.; Kiladjian, J.-J.; Gotlib, J.; Cervantes, F.; Mesa, R.A.; Sarlis, N.J.; Peng, W.; Sandor, V.; Gopalakrishna, P.; et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica 2015, 100, 1139–1145. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chen, C.; Kundra, A.; Kodali, S.; Pandey, A.; Wong, C.; Cheung, T.; Gotlieb, V.; Joseph, G.; Tribie, S. Programmed Cell Death Receptor (PD-1) Ligand (PD-L1) expression in Philadelphia chromosome-negative myeloproliferative neoplasms. Leuk. Res. 2019, 79, 52–59. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014, 5, 12189–12202. [Google Scholar] [CrossRef]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334. [Google Scholar] [CrossRef]
- Fujimura, T.; Ring, S.; Umansky, V.; Mahnke, K.; Enk, A.H. Regulatory T Cells Stimulate B7-H1 Expression in Myeloid-Derived Suppressor Cells in ret Melanomas. J. Investig. Dermatol. 2012, 132, 1239–1246. [Google Scholar] [CrossRef]
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 783. [Google Scholar] [CrossRef]
- Weber, R.; Fleming, V.; Hu, X.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Meyer, C.; Cagnon, L.; Costa-Nunes, C.M.; Baumgaertner, P.; Montandon, N.; Leyvraz, L.; Michielin, O.; Romano, E.; Speiser, D.E. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 2014, 63, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Martens, A.; Zelba, H.; Stutz, C.; Derhovanessian, E.; Di Giacomo, A.M.; Maio, M.; Sucker, A.; Schilling, B.; Schadendorf, D.; et al. Myeloid-Derived Suppressor Cells Predict Survival of Patients with Advanced Melanoma: Comparison with Regulatory T Cells and NY-ESO-1- or Melan-A–Specific T Cells. Clin. Cancer Res. 2014, 20, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Soda, H.; Ogawara, D.; Fukuda, Y.; Tomono, H.; Okuno, D.; Koga, S.; Taniguchi, H.; Yoshida, M.; Harada, T.; Umemura, A.; et al. Dynamics of blood neutrophil-related indices during nivolumab treatment may be associated with response to salvage chemotherapy for non-small cell lung cancer: A hypothesis-generating study. Thorac. Cancer 2019, 10, 341–346. [Google Scholar] [CrossRef]
- Feng, J.; Chen, S.; Li, S.; Wu, B.; Lu, J.; Tan, L.; Li, J.; Song, Y.; Shi, G.; Shi, Y.G.; et al. The association between monocytic myeloid-derived suppressor cells levels and the anti-tumor efficacy of anti-PD-1 therapy in NSCLC patients. Transl. Oncol. 2020, 13, 100865. [Google Scholar] [CrossRef]
- Northwestern University. A Phase 1, Single Arm, Single Center Pilot Study of Medi4736, an Anti-Pdl1 Therapy, for Patients with Myelofibrosis. clinicaltrials.gov. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT02871323 (accessed on 28 March 2023).
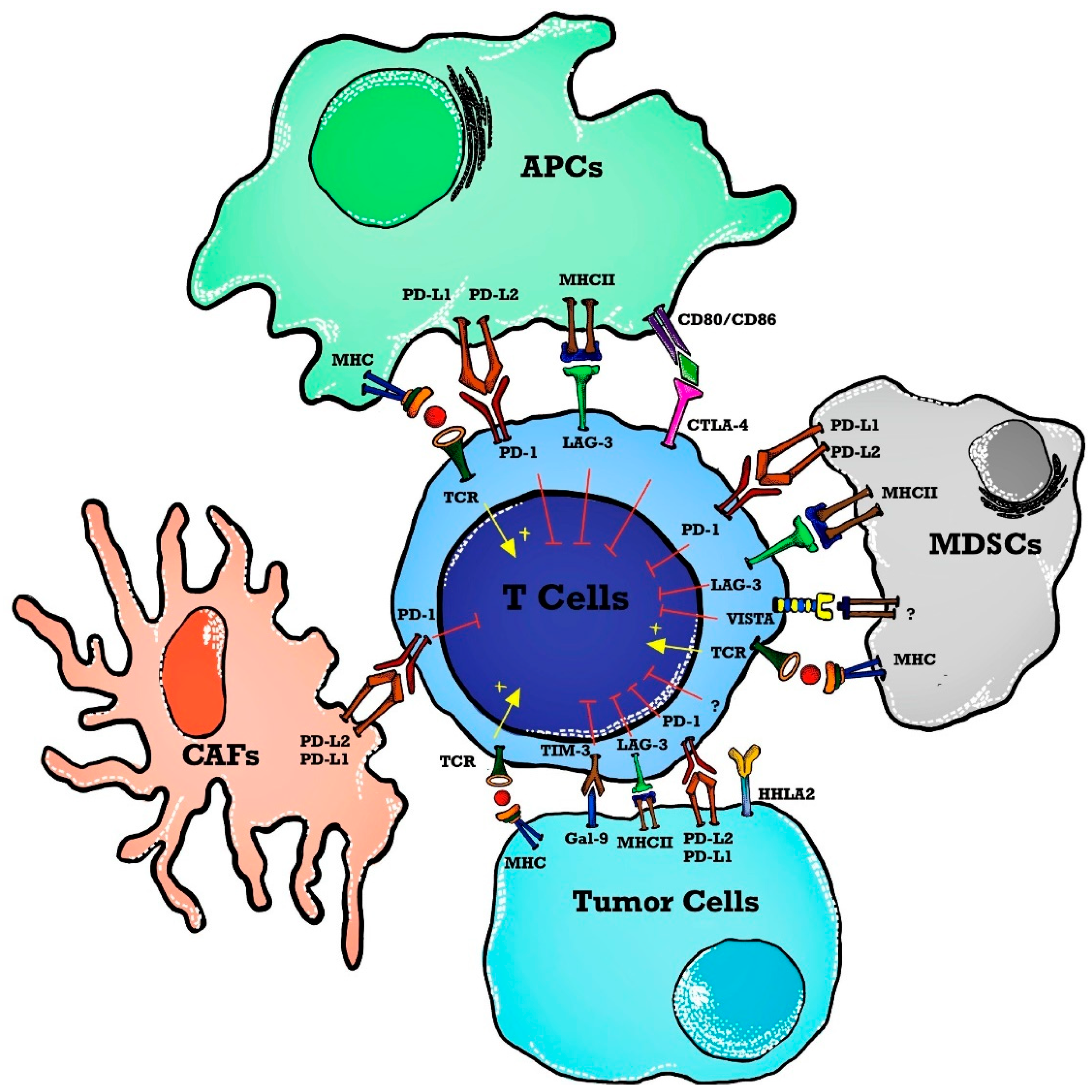
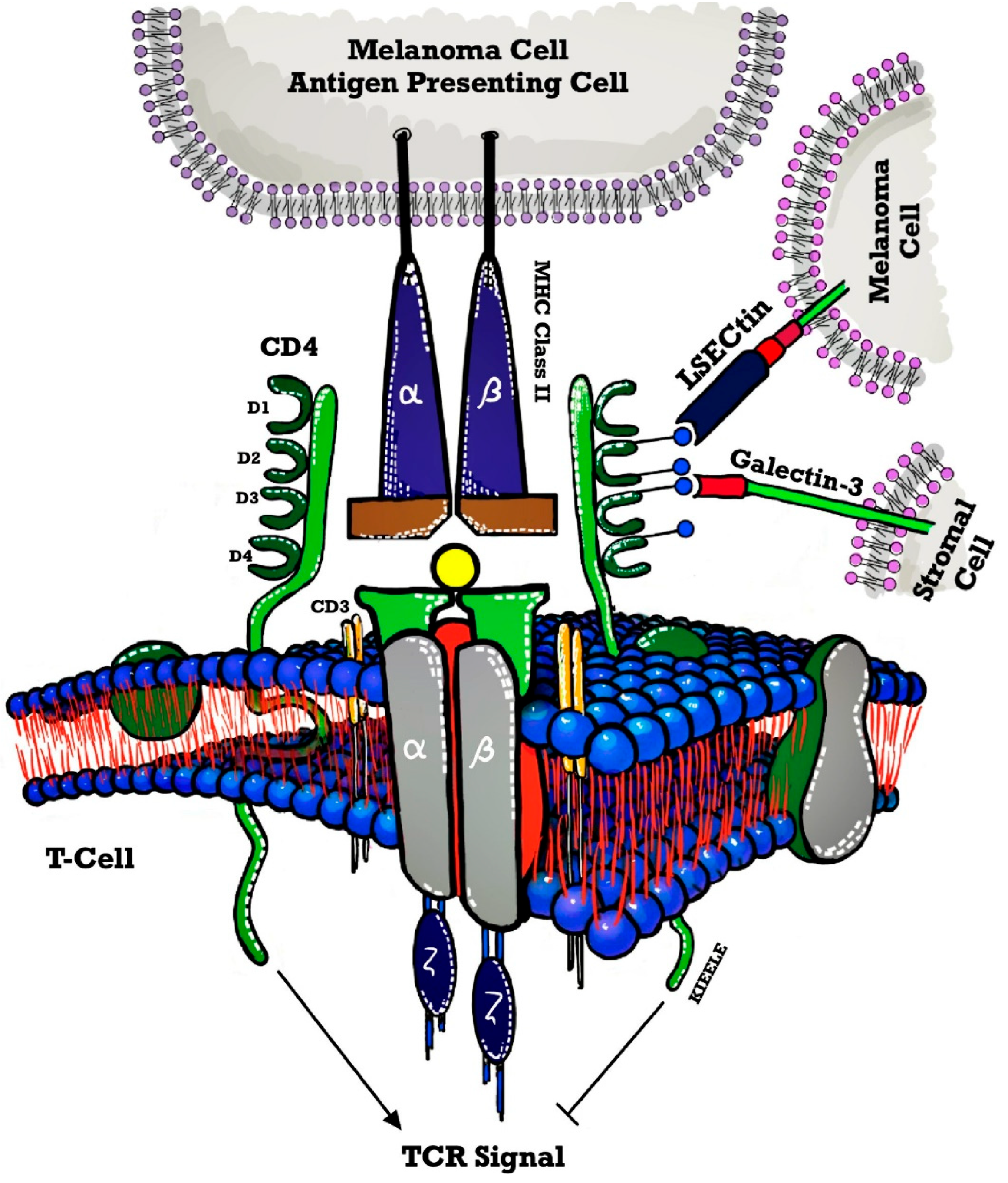
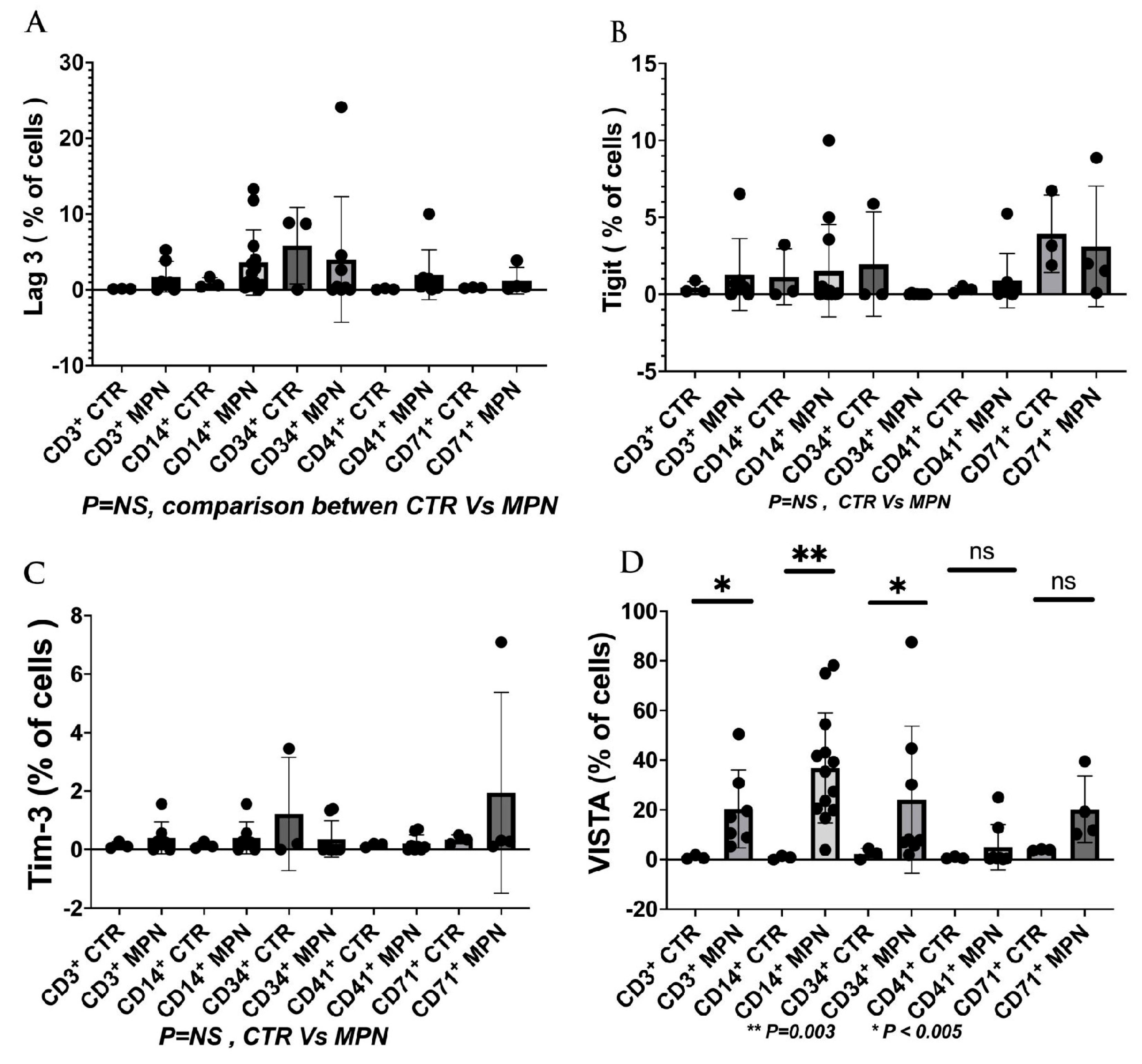

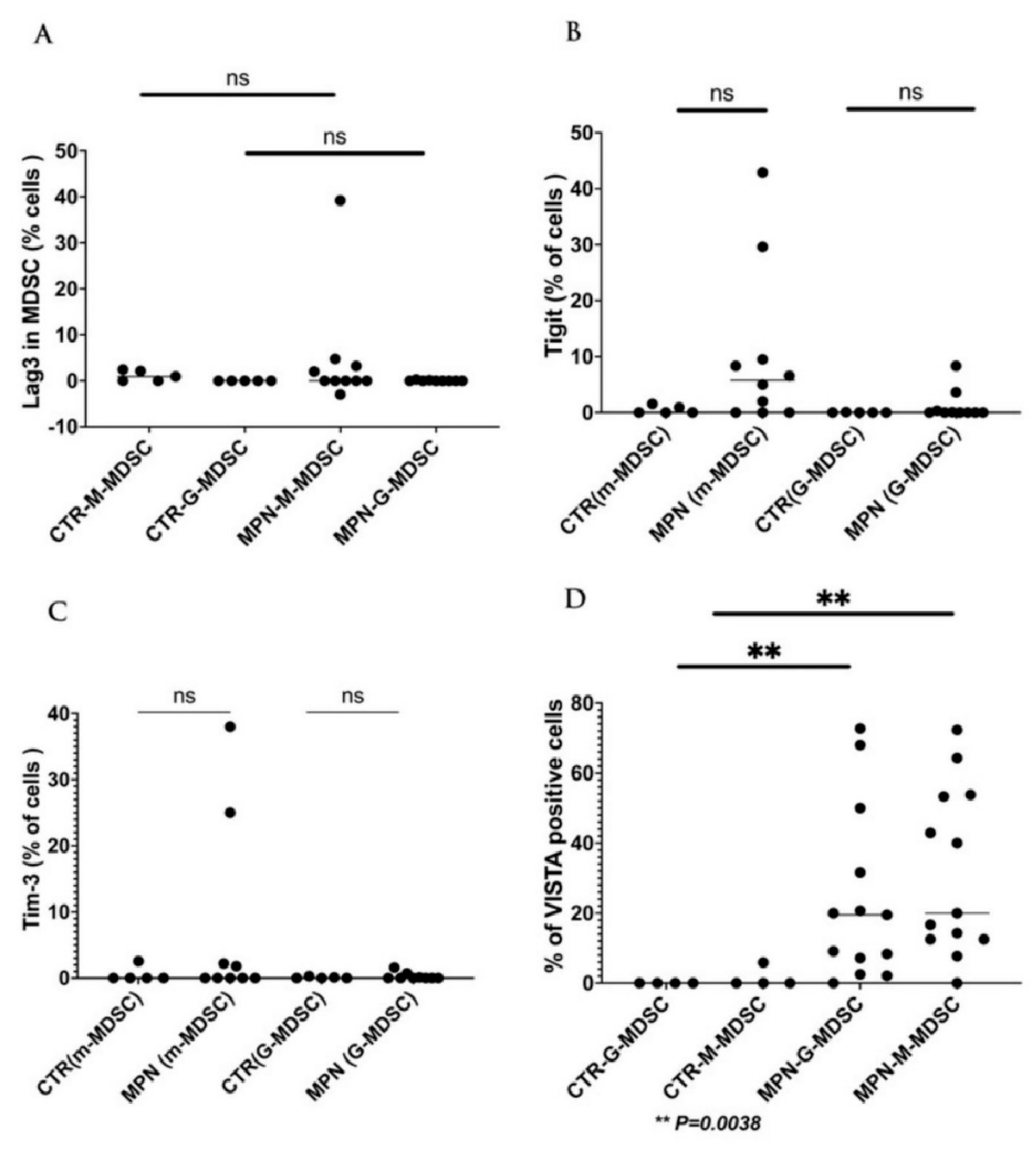
| NCT Number | Sponsors/Collaorators | Title | Drug Name | Phase | Tumor/Disease | Status | Outcome Measures | ICI Type | Study Designs |
|---|---|---|---|---|---|---|---|---|---|
| NCT04566978 | Memorial Sloan Kettering Cancer Center | 89Zr-DFO-REGN3767 in PET Scans in People With Diffuse Large B Cell Lymphoma (DLBCL) | Drug: 89Zr-DFO-REGN3767|Diagnostic Test: PET/CT | Early Phase 1 | Large B-cell Lymphoma|DLBCL | Recruiting | Biodistribution of 89Zr-DFO-REGN3767|Optimal 89Zr-DFO-REGN3767 mass dose for tumor targeting|Optimal time for imaging and tumor uptake post 89Zr-DFO-REGN3767 administration|Tumor lesion uptake of 89Zr-DFO-REGN3767 and correlate with LAG-3 expression by IHC | LAG-3 | Allocation: Randomized|Intervention Model: Sequential Assignment|Masking: None (Open Label)|Primary Purpose: Diagnostic |
| NCT03310619 | Celgene | A Safety and Efficacy Trial of JCAR017 Combinations in Subjects With Relapsed/Refractory B-cell Malignancies (PLATFORM) | Biological: JCAR017|Drug: Durvalumab|Drug: CC-122|Drug: Ibrutinib|Drug: CC-220|Drug: Relatlimab|Drug: Nivolumab|Drug: CC-99282 | Phase 1|Phase 2 | Lymphoma, Non-Hodgkin|Lymphoma, Large B-Cell, Diffuse|Lymphoma, Follicular | Completed | Dose-limiting toxicity (DLT) rates|Complete Response Rate|Adverse Events (AEs)|Progression-free survival (PFS)|Overall survival (OS)|Overall response rate (ORR)|Duration of response (DOR)|Event-free survival (EFS)|Pharmacokinetic (PK)-Cmax|Pharmacokinetic (PK)-Tmax|Pharmacokinetic (PK)-AUC|Health-related quality of life (HRQoL)|Quality of Life C30 questionnaire (EORTC-QLQ-C30)|European Quality of Life-5 Dimensions health state classifier to 5 Levels (EQ-5D-5L) | LAG-3 | Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT02061761 | Bristol-Myers Squibb | A Phase 1/2a Dose Escalation and Cohort Expansion Study of the Safety, Tolerability, and Efficacy of Anti-LAG-3 Monoclonal Antibody (Relatlimab, BMS-986016) Administered Alone and in Combination With Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Relapsed or Refractory B-Cell Malignancies | Biological: BMS-986016|Biological: BMS-936558 | Phase 1|Phase 2 | Hematologic Neoplasms | Completed | Safety measured by the rate of Adverse events (AEs), Serious Adverse events (SAEs), death and laboratory abnormalities [Time Frame: Up to approximately 2.3 years] | LAG-3 | Allocation: Non-Randomized|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT03598608 | Merck Sharp & Dohme LLC | A Phase 1/Phase 2 Clinical Study to Evaluate the Safety and Efficacy of a Combination of MK-4280 and Pembrolizumab (MK-3475) in Participants With Hematologic Malignancies | Biological: pembrolizumab|Biological: Favezelimab | Phase 1|Phase 2 | Hodgkin Disease|Lymphoma, Non-Hodgkin|Lymphoma, B-Cell | Recruiting | Percentage of Participants Experiencing a Dose-limiting Toxicity (DLT); Percentage of Participants Experiencing an Adverse Event (AE); Percentage of Participants with Treatment Discontinuations Due to an AE | LAG-3 | Allocation: Non-Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT04913922 | Ludwig-Maximilians-University of Munich | An Open-Label Phase II Study of Relatlimab (BMS-986016) With Nivolumab (BMS-936558) in Combination With 5-Azacytidine for the Treatment of Patients With Refractory/Relapsed Acute Myeloid Leukemia and Newly Diagnosed Older Acute Myeloid Leukemia Patients | Drug: Azacitidine Injection|Drug: Nivolumab|Drug: Relatlimab | Phase 2 | Acute Myeloid Leukemia | Recruiting | Maximum tolerated dose (MTD); Dose-limiting toxicities (DLTs); Objective response rate (ORR) | LAG-3 | Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT03365791 | Novartis Pharmaceuticals | Modular Phase 2 Study to Link Combination Immune-therapy to Patients With Advanced Solid and Hematologic Malignancies. Module 9: PDR001 Plus LAG525 for Patients With Advanced Solid and Hematologic Malignancies. | Biological: PDR001; Biological: LAG525 | Phase 2 | Small cell lung cancer, Gastric/esophageal adenocarcinoma, Castration resistant prostate adenocarcinoma (CRPC), Soft tissue sarcoma, Ovarian adenocarcinoma, Advanced well-differentiated neuroendocrine tumors, Diffuse large B cell lymphoma (DLBCL). | Completed | Clinical Benefit Rate (CBR) at 24 Weeks of PDR001+LAG525 by Tumor Type in Multiple Solid Tumors and Lymphoma | LAG-3 | Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT Number | Sponsors/Collaorators | Title | Drug Name | Phase | Tumor/Disease | Status | Outcome Measures | ICI Type | Study Designs |
|---|---|---|---|---|---|---|---|---|---|
| NCT05315713 | Hoffmann-La Roche | A Phase Ib/II Open-Label, Multicenter Study Evaluating the Safety, Efficacy, and Pharmacokinetics of Mosunetuzumab in Combination With Tiragolumab With or Without Atezolizumab in Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma | Mosunetuzumab SC; Tiragolumab; Atezolizumab; Tocilizumab | Phase 1 and 2 | Relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma (FL) | Active, not recruiting | Percentage of Participants with Adverse Events; Best Objective Response Rate (ORR) | TIGIT | Allocation: Non-randomized|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT04045028 | Genentech, Inc. | A Phase Ia/Ib Open-Label, Multicenter Study Evaluating the Safety and Pharmacokinetics of Tiragolumab as a Single Agent and in Combination With Atezolizumab and/or Daratumumab in Patients With Relapsed or Refractory Multiple Myeloma, and as a Single Agent and in Combination With Rituximab in Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma | Tiragolumab; Daratumumab/rHuPH20; Rituximab; Atezolizumab | Phase 1 | Relapsed or Refractory Multiple Myeloma, Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma | Terminated (slow recruitment) | Percentage of Participants With Adverse Events; Objective Response Rate (ORR) for R/R MM; ORR for R/R NHL; Percentage of Participants With Anti-Drug Antibodies (ADAs) to Tiragolumab/Atezolizumab | TIGIT | Allocation: Non-randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT05267054 | BeiGene | A Phase 1b/2 Study Investigating the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of Ociperlimab (BGB A1217) in Combination With Tislelizumab (BGB A317) or Rituximab in Patients With Relapsed or Refractory Diffuse Large B Cell Lymphoma | Ociperlimab; Tislelizumab; Rituximab | Phase 1 and 2 | Relapsed Diffuse Large B-cell Lymphoma | Recruiting | Number of participants with adverse events (AEs); Recommended Phase 2 dose (RP2D) of ociperlimab when administered in combination with tislelizumab or rituximab; ORR; CRR; DOR; TTR; PFS; OS; Host immunogenecity | TIGIT | Allocation: Non-randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT04150965 | Multiple Myeloma Research Consortium | A Phase I/II Assessment of Combination Immuno-Oncology Drugs Elotuzumab, Anti-LAG-3 (BMS-986016) and Anti-TIGIT (BMS-986207) | Elotuzumab, pomalidomide, dexamethasone; Anti-LAG-3; Anti-LAG-3 + Pomalidimide + Dexamethasone; Anti-TIGIT; Anti-TIGIT + Pomalidimide + Dexamethasone | Phase 1 and 2 | Relapsed Diffuse Large B-cell Lymphoma; Multiple Myeloma | Recruiting | Overall Response Rate; Frequency, type and grade of Adverse Events and Serious Adverse Events | TIGIT | Allocation: Randomized|Intervention Model: Sequential Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT05005442 | Merck Sharp & Dohme LLC | A Phase 2, Open-label Study to Evaluate the Safety and Efficacy of MK-7684A (MK-7684 [Vibostolimab] With MK-3475 [Pembrolizumab] Coformulation) in Participants With Relapsed or Refractory Hematological Malignancies | Biological: Pembrolizumab/vibostolimab coformuation | Phase 2 | Hematological Malignancies | Recruiting | Number of Participants with a Dose-Limiting Toxicity (DLT); Number of Participants Who Experienced an Adverse Event (AE); ORR, DOR, DCR | TIGIT | Allocation: NA|Intervention Model: Single group assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT04354246 | Compugen Ltd | A Phase 1 Study of The Safety and Tolerability of COM902 in Subjects With Advanced Malignancies | COM902 monotherapy; COM902 in combination with COM701 (both at the RDFE); Triplet combination of COM902 + COM701 + Pembrolizumab. | Phase 1 | Advanced cancer; ovarian cancer; lung cancer; plasma cell neoplasm; MM; HNSCC; Microsatellite stabel colorectal carcinoma; MSS-CRC | Recruiting | The safety and tolerability of COM902 monotherapy; To identify the maximum tolerated dose (MTD) and/or recommended Phase 2 dose (RP2D); To characterize the pharmacokinetic (PK) profile of COM902 as monotherapy in subjects with advanced malignancies; | TIGIT | Allocation: Non-randomized|Intervention Model: Sequential Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
| NCT04254107 | Seagen Inc. | A Phase 1 Study of SEA-TGT (SGN-TGT) in Subjects With Advanced Malignancies | SEA-TGT; sasanlimab; brentuximab vedotin | Phase 1 | NSCLC; Gastric carcinoma; Gastroesophageal Junction Carcinoma; classic HL; DLBCL; Peripheral T-cell Lymphoma; Cutaneous Melanoma; Head and Neck Squamous Cell Carcinoma; Bladder carcinoma; ovarian cancer; triple negative breast cancer; cervical cancer | Recruiting | Number of participants with adverse events (AEs); Number of participants with laboratory abnormalities by grade; Number of participants with a dose-limiting toxicity (DLT) at each dose level | TIGIT | Allocation: Non-randomized|Intervention Model: Sequential Assignment|Masking: None (Open Label)|Primary Purpose: Treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.; Hakobyan, N.; Wang, J.-C. Role of Next Generation Immune Checkpoint Inhibitor (ICI) Therapy in Philadelphia Negative Classic Myeloproliferative Neoplasm (MPN): Review of the Literature. Int. J. Mol. Sci. 2023, 24, 12502. https://doi.org/10.3390/ijms241512502
Yadav R, Hakobyan N, Wang J-C. Role of Next Generation Immune Checkpoint Inhibitor (ICI) Therapy in Philadelphia Negative Classic Myeloproliferative Neoplasm (MPN): Review of the Literature. International Journal of Molecular Sciences. 2023; 24(15):12502. https://doi.org/10.3390/ijms241512502
Chicago/Turabian StyleYadav, Ruchi, Narek Hakobyan, and Jen-Chin Wang. 2023. "Role of Next Generation Immune Checkpoint Inhibitor (ICI) Therapy in Philadelphia Negative Classic Myeloproliferative Neoplasm (MPN): Review of the Literature" International Journal of Molecular Sciences 24, no. 15: 12502. https://doi.org/10.3390/ijms241512502
APA StyleYadav, R., Hakobyan, N., & Wang, J.-C. (2023). Role of Next Generation Immune Checkpoint Inhibitor (ICI) Therapy in Philadelphia Negative Classic Myeloproliferative Neoplasm (MPN): Review of the Literature. International Journal of Molecular Sciences, 24(15), 12502. https://doi.org/10.3390/ijms241512502






